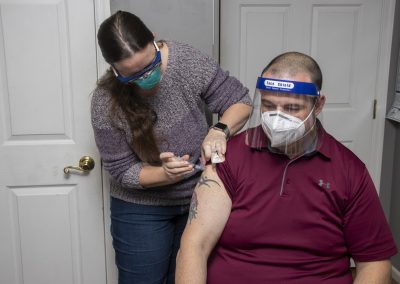
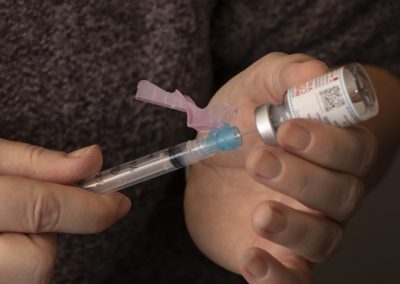
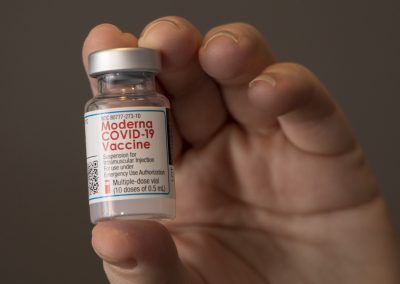
The Covid-19 Vaccine
The vaccine distribution plan created by Governor Cuomo’s office has delegated that people with developmental disabilities living in congregate settings and the staff who care for them are included in phase 1a, and will be among the first to receive access to the Covid-19 vaccine. At Springbrook, we strive to give you the most trusted and up-to-date information about the Covid-19 pandemic and this newly developed vaccine.
People get vaccinated so it is easier to fight diseases like Covid-19. Vaccines are just one of the ways to help stop (or slow) the spread of disease. The information on this page is intended to help you make informed decisions about whether or not you choose to be vaccinated. As always, please discuss the vaccine with your healthcare provider before making any decision about your health.
COVID-19 Screening & Consent Form
Families are required to complete a consent form in order for their loved ones to receive the COVID-19 vaccine. Please reference the list below for your residence contact.
Consent Form Contact List
Hayes, Oxford, Nichols, South Broad Street: Ketharine Safavizadeh
Glensbridge, Grandview, Morris, Sherburne, West Oneonta: Heather Anderson
Cook Street, Pierstown, Stone Quarry: Heather Schneider
Kelly Corners, Milford, Pines, Swart Hollow: Molly Steenland
Ford Ave, Oak & Elm, Otego, Walnut: Amanda Steinert
Hilltop, Highland: Bobbie Martindale
Snell, Sunshine, Susquehanna, Taylor: Kim MacPherson
LeChase, Scriven, Wright: Isabelle Brundege-Wood
Covid and People with I/DD
A recent review of private health insurance claims data for 467,773 people diagnosed with the coronavirus in the U.S. between April and August found that individuals with developmental disorders were three times more likely to die compared to others. That’s the highest risk of any of the 15 conditions that the study looked at, which included heart failure, chronic kidney disease, liver disease, and various types of cancer. Meanwhile, a separate category for people with intellectual disabilities and related conditions like Down syndrome showed a risk of death 2.75 times higher and was third on the list of conditions. That was followed by spina bifida and other nervous system anomalies. You can read more about this report here.
We also encourage you to read this important letter from the OPWDD Commissioner.

Vaccine Webinar
On December 22, Springbrook held an informative webinar to discuss the COVID-19 vaccines that have received emergency use authorization. CEO Patricia Kennedy, COO Seth Haight, and CCO Katherine Ramiza hosted the webinar and answered questions for viewers. If you were unable to attend the webinar, we encourage you to watch it here.
2020-VaccineInfo-Family from Springbrook NY on Vimeo.

Latest Information
The Pfizer BioNTech (pronounced Bee-On-Tech) vaccine has received emergency use authorization by the FDA as of 12/11/2020.
The Moderna (pronounced Mo-der-Na) vaccine has received emergency use authorization by the FDA as of 12/18/2020. Please take a look at this supplied Vaccine Recipient Fact Sheet we received directly from Moderna.
Both vaccines are mRNA (messenger RNA) vaccines meaning they do not inject the patient with a live or dead strain of the virus.
-
- mRNA vaccines teach your body to create virus proteins. The immune system develops antibodies against these proteins to help you fight the virus that causes COVID in the case that you are exposed (immune response).
Both vaccines are completed with two doses given 3-4 weeks apart depending on which vaccine you get.
No studies with the vaccine have been completed in children under 12 and limited information is available on pregnant women.
The vaccine IS being recommended for people who have already had COVID.
Patients who choose to get the vaccine will still be required to wear a mask and social distance.
The vaccine will be available at NO COST to all New Yorkers.
First priority in NY to get the vaccine are:
-
- High-Risk Hospital Workers (ER, ICU & Pulmonary Dept. Staff)
- Nursing Home Residents & Staff
- All Long-Term and Congregate Care Residents & Staff (Including OPWDD)
- EMS workers
- Other healthcare workers
- Coroners & medical examiners
NY has launched a website about the vaccine which will host the most up to date vaccine information: https://covid19vaccine.health.ny.gov/

Additional Videos
Bassett Healthcare Network sponsored a public town hall on December 17, 2020 to help our local communities better understand the Pfizer and Moderna COVID-19 vaccines. Our team answered some frequently asked questions about these vaccines, including their safety and effectiveness, as well as addressed some common myths and concerns about the vaccines. If you missed this webinar, please watch this recording.
Frequently Asked Questions
Can I get Covid-19 from the vaccine?
You cannot get Covid-19 from the vaccine. None of the leading vaccines (Pfizer, Moderna, AstraZeneca, Janssen) contain the actual SARS-CoV-2 virus. The vaccine uses messenger RNA (mRNA), the genetic material that our cells read to make proteins. Once the proteins, which don’t cause disease, are produced, the body launches an immune response against the virus, enabling the person to develop immunity.
This recent article in the New York Times gives a great overview of how mRNA vaccines work.
Can I choose which vaccine I get?
This depends on a number of factors, including the supply in your area at the time you’re vaccinated and whether certain vaccines are found to be more effective in certain populations, such as older adults.
Is there a risk that supply will run out before I get my second dose?
If you are in the first group of people vaccinated, your booster shot will be set aside for you and won’t be given to someone else. Later, when supplies are more plentiful, reserves probably won’t be necessary. Gen. Gustave F. Perna, the chief operating officer for Operation Warp Speed (the federal effort to speed a vaccine to market) has said that after the first doses are given, the doses earmarked for the second shot will be set aside to be given three weeks later. An additional 500,000 backup doses will also be held in reserve, in case they are unexpectedly needed.
How long will it take to work?
You won’t get the full protection from the Pfizer-BioNTech vaccine until about a week after the second dose, based on clinical trial data. The researchers found that the vaccine’s protection started to emerge about ten days after the first dose, but it only reached 52 percent efficacy, according to a report in the New England Journal of Medicine. A week after the second dose, the efficacy rose to 95 percent.
What should I mention to my provider before I receive any shots?
It is recommended that you inform your vaccination provider about all medical conditions, including if you:
- Have any allergies
- Have a fever
- Have a bleeding disorder or are on a blood-thinner
- Are immunocompromised or are taking a medicine that affects your immune system
- Are pregnant or are planning to become pregnant
- Are breastfeeding
- Have received another Covid-19 vaccine
I had Covid-19 already. Should I still get vaccinated?
It’s safe, and probably even beneficial, for anyone who has had COVID-19 to get the vaccine at some point, experts said. Although people who have contracted the virus do have immunity, it is too soon to know how long it lasts. So for now, it makes sense for them to get the shot. The question is when. Some members of the CDC advisory committee have suggested people who have had COVID-19 in the past 90 days should be toward the back of the line.
If I have allergies, should I be concerned?
People with severe allergies who have experienced anaphylaxis in the past should talk to their doctors about how to safely get the vaccine and what precautions to take. The FDA. Has said it would require Pfizer to increase its monitoring for anaphylaxis and submit data on it once the vaccine comes into use. Fewer than one in a million recipients of other vaccines a year in the U.S. have an anaphylactic reaction, said Dr. Paul Offit, a vaccine expert at Children’s Hospital of Philadelphia. Among those who participated in the Pfizer trials, a very small number of people had allergic reactions. A document published by the FDA said that 0.63 percent of participants who received the vaccine reported potential allergic reactions, compared to 0.51 percent of people who received a placebo. In Pfizer’s late-stage clinical trial, one of the 18,801 participants who received the vaccine had an anaphylactic reaction, according to safety data published by the FDA on December 10, 2020. None in the placebo group did.
Additional Sources:
New York Times – Here’s What People With Allergies Should Know About Covid Vaccines
How do I report a problem or bad reaction to getting vaccinated?
The CDC and FDA encourage the public to report possible side effects (called adverse events) to the Vaccine Adverse Event Reporting System (VAERS). This national system collects these data to look for adverse events that are unexpected, appear to happen more often than expected, or have unusual patterns of occurrence. Healthcare providers will be required to report certain adverse events following vaccination to VAERS. Healthcare providers also have to adhere to any revised safety reporting requirements according to FDA’s conditions of authorized use throughout the duration of any Emergency Use Authorization; these requirements would be posted on FDA’s website.
The CDC is also implementing a new smartphone-based tool called v-safe to check-in on people’s health after they receive a COVID-19 vaccine. When you receive your vaccine, you should also receive a v-safe information sheet telling you how to enroll in v-safe. If you enroll, you will receive regular text messages directing you to surveys where you can report any problems or adverse reactions you have after receiving a COVID-19 vaccine.